Design according to cGMP / EP / USP
Good Manufacturing Practices (GMP) are the working standards required to align with the instructions recommended by the agencies that control authorizations and licenses for the production and sale of medicines and active pharmaceutical or food products. These standards set out minimum requirements for a pharmaceutical or food manufacturer to meet in order to ensure that they are of high quality and do not pose a risk to the consumer or the target public.
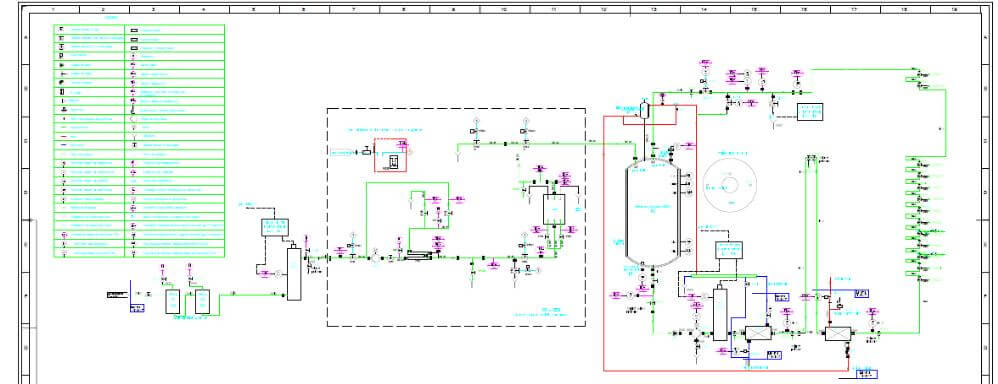
Design and execution of purified water recirculation loops using orbital welding
The distribution loop contains pneumatic valves, heat exchangers, conductivity, temperature, pressure and flow / speed, TOC sensors, parameters continuously monitored by the PLC in the automation panel.
For pipes and fittings in contact with purified water we use AISI316L stainless steel, Ra 0.8 … 0.5 µm, joined by orbital welding.
Automation and monitoring of functional parameters
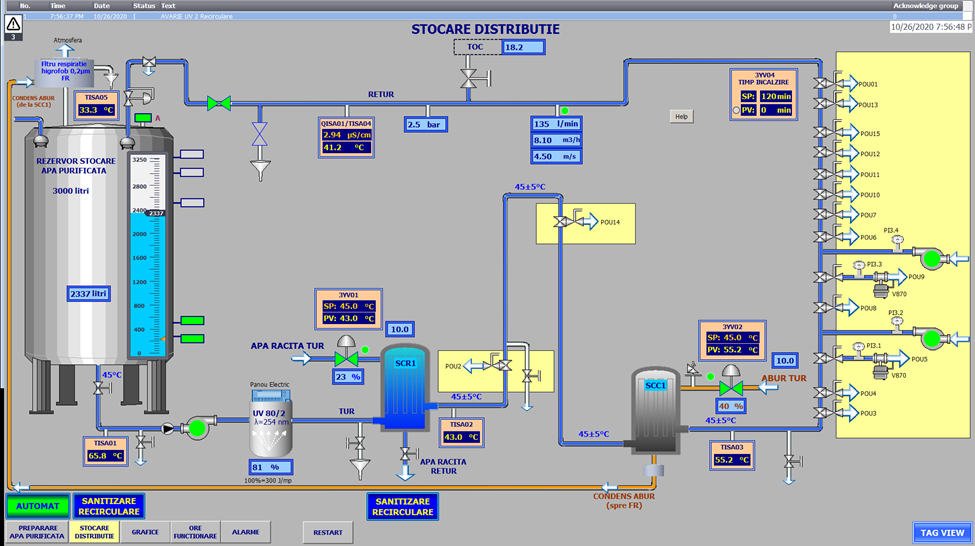
Monitoring the water purification system is designed to meet our goal of providing facilities that are part of the concept of quality management „zero defects”. In this way, operating errors can be avoided and significant savings in financial resources are made through an operation that eliminates the possibility of producing water below the required quality standards.
The monitoring is performed by means of the PLC programmable controller which has the role of control and execution for the automation elements placed in the key points of the system. Basically, the operator can follow the set state parameters of the water purification system by following a single panel that will provide him with all the essential operating data, as well as the immediate remedial solutions in case of failure or error.
The control panel offers the possibility to set the following parameters:
- The quality of the water treated before entering the purification plant
- Purified water quality
- The amount of purified water produced
- The quality of water stored and circulated through the recirculation loop
- Purified water temperature
- Thermal sanitation of the purified water storage and distribution section, respectively of the RO-EDI section
- The recorded data can be viewed on an Open Office compatible program.
- The data will be presented in the form of a table indicating the date and time of recording each parameter and each alarm in one minute.
- Non-editable database with sufficient memory for 10 years of operation.
- Idem, it will not allow it to be erased or influenced by any disturbance in the operation of the automation panel
- The operating software is validated according to the most demanding norms in CFR21 part 11
- Dedicated program AUDIT TRAIL

 Română
Română